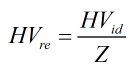C263 – ASTM D3588/GPA 2145 – Calorific Value and Relative Density
Description
This calculates the calorific value, compressibility factor and relative density of a natural gas mixture. The calculations are carried out at the standard US base temperature of 60°F.
References
ASTM D3588-98: Standard Practice for Calculating Heat Value, Compressibility Factor and Relative Density of Gaseous Fuels (1998)
GPA 2145: Table of Physical Properties for Hydrocarbons and Other Compounds of Interest to the Natural Gas Industry (1989, 2000, 2003, 2009)
GPA TP-17: Table of Physical Properties of Hydrocarbons for Extended Analysis of Natural Gases (1998)
Kelton calculation reference C263
FLOCALC calculation reference F067
KIMS calculation reference K202
Note: The BTU referred to in this calculation is the international table BTU, BTU = 1055.056 J.
Options
Gas Analysis
- Extended Analysis
This option allows common trace gases to be included in the analysis.
- Refinery trace gases
This option allows refinery trace gases to be included in the analysis.
GPA 2145 Physical Constants Table
- 1989
- 2000
- 2003
- 2009
This option allows the user to select which revision of GPA 2145 they wish to take the table of physical constants, associated with the component gases, from.
Wet Gas Correction
- Apply
This option allows the user to correct for wet gas
Mole Percent of Water
- User entered
- Raoult’s law approximation
This allows the user to enter the mole percent of water or have it calculated according to Raoult’s Law.
Constants
Gas constant – R = 8.314472 Jmol-1K-1
Vapour pressure of water – Pw_vap = 0.25636 psi
Base Temperature – Tb = 60°F
Calculation
Compressibility Factor
The compressibility factor is calculated by:
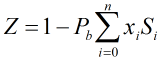
| Where | ||
|---|---|---|
| Pb | = | Base pressure |
| xi | = | Mole fraction of ith gas component |
| Si | = | Summation factor of ith gas component |
Relative Density
The relative density is calculated by:
Ideal
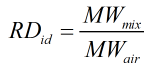
Real
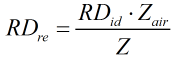
| Where | ||
| MWmix | = | Molecular weight of gas mixture |
| MWair | = | Molecular weight of air |
| Z | = | Compressibility factor of gas mixture |
| Zair | = | Compressibility factor of air |
Raoult’s Law approximation of water content
Assuming Raoult’s law, the water content when the gas is saturated will be:
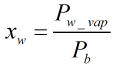
Volumetric Heating Value
The heating value is calculated using component ideal volumetric heating values if base pressure is equal to 14.696 psi
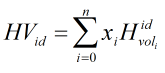
If base pressure is not 14.696 psi the calculation multiplies the component ideal volumetric heating values by the pressure ratio (in psi) Pb/14.696 and then carries out the above calculation.
If there is a wet gas correction based on the Raoult’s law approximation then ideal volumetric heating value is calculated by:
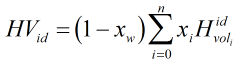
| Where | ||
| xi | = | Mole fraction of ith gas component |
| MWi | = | Molecular weight of ith gas component |
| Hvolidi | = | Ideal volumetric gross heating value of ith gas component |
These ideal heating values are divided by the compressibility factor to give a “real” heating value. This is not the real heating value but is actually the ideal heating value per real cubic foot.