C180 – COSTALD-Tait Density Calculation
Description
This COSTALD calculation comprises four distinct density calculation options.
The “standard” COSTALD equation is used to calculate the saturated liquid density of light hydrocarbon mixtures (LPGs) from composition.
The “enhanced” COSTALD equation, which differs only slightly from the “standard” COSTALD equation, is used to calculate the saturated liquid density of LNG mixtures (i.e. predominantly CH4).
The Tait extension to the COSTALD equation (known as COSTALD-Tait) calculates the compressed liquid density of light hydrocarbon mixtures (i.e. density at pressures above the saturation pressure). The Tait extension applies to both the “standard” and “enhanced” COSTALD equations giving four options in total.
References
IP Petroleum Manual. Part XII – Static and Dynamic Measurement of Light Hydrocarbon Liquids. Section 1 – Calculation Procedures. [1]
ISO 6578:1991 (BS 7577:1992) – Annex G. [2]
KELTON calculation reference C180
FLOCALC calculation reference F059
KIMS calculation references K201
Calculations
a) “Standard” COSTALD The LPG mixture density is calculated from the following equation:

| Where | ||
| ρt | = | Saturated mixture density at temperature t |
| xi | = | Mole fraction of ith component |
| Mi | = | Molar mass of ith component |
| VS | = | Molar volume of mixture at temperature t |
The molar mass values are obtained from Ref [1] above. The molar volume of the mixture, VS, is calculated from:
![]()
| Where | ||
| V’m | = | Characteristic volume of the mixture |
| VoR | = | Corresponding states function for normal fluids |
| Vδ R | = | Deviation function |
| ωm | = | Acentric factor of mixture |
The above parameters are calculated as follows:
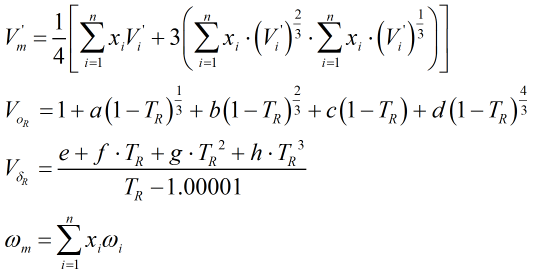
| Where | ||
| V’i | = | Characteristic volume of ith component |
| TR | = | Reduced temperature of the mixture |
| ωi | = | Acentric factor of ith component |
The constants a, b, c, d, e, f, g and h, along with the component characteristic volumes and acentric factors, are obtained from Ref [1] above. The reduced temperature, TR, is calculated from:
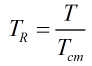
| Where | ||
| T | Absolute temperature of mixture | |
| Tcm | Critical temperature of the mixture |
The critical temperature of the mixture is calculated from:
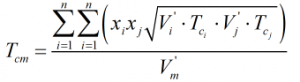
| Where | ||
| Tci | = | Critical temperature of ith component |
| Tcj | = | Critical temperature of jth component |
| V’i | = | Characteristic volume of ith component |
| V’j | = | Characteristic volume of jth component |
The component critical temperatures are obtained from Ref [1] above.
b) “Enhanced” COSTALD The LNG mixture density is calculated using the same method as the “standard” COSTALD equation above (for LPG), with the only exception being the calculation of the critical temperature of the mixture, Tcm. For the “enhanced” COSTALD equation (for LNG only), Tcm is calculated from:
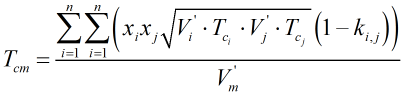
where the additional interaction parameters, ki,j, are given in Ref [1].
c) COSTALD-Tait The compressed liquid density of light hydrocarbon mixtures is calculated from the following equation:
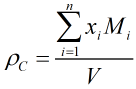
where V is the compressed molar volume of the mixture given by:
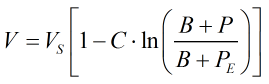
| Where | ||
| VS | = | Saturated molar volume from the main COSTALD routine |
| P | = | Observed pressure |
| PE | = | Equilibrium vapour pressure |
The constants B and C are calculated from:
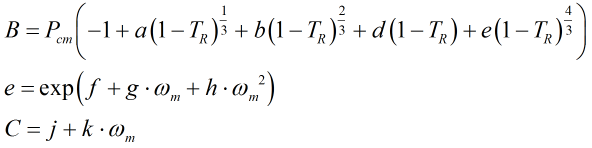
where Pcm is the critical pressure of the mixture, the derivation of which is given further below. The constants a, b, d, f, g, h, j, and k are given in Ref [1] above.
NOTE: Ref [1] contains an error in stating “d” as 135.1102 (+ve), whereas the original publication from which the equations are taken give “d” as -135.1102 (-ve).
The equilibrium vapour pressure, PE, is calculated from:
![]()
where the reduced pressure, PR is given by:
![]()
and
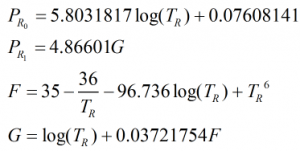
and Pcm is given by:
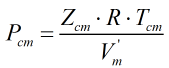
| Where | ||
| Zcm | = | Critical compressibility factor of the mixture |
| R | = | Gas constant equal to 8.31441 J·mol-1·K-1 |
| Tcm | = | Critical temperature of the mixture from the main COSTALD routine |
| V’m | = | Characteristic volume of the mixture from the main COSTALD routine |
The critical compressibility factor is given as:
![]()